The Determination Of Fluoride In Urine
The technique used in this procedure is that of a single known addition to minimise sample matrix effects which cause problems with the direct potentiometric approach.
Equipment Required
1. EDT directION DR359TX Ion Meter
2. 3221 Fluoride Combination ISE
3. 21333 Fluoride Standard Solution 1000ppm
4. 30333 Fluoride Buffer (TISAB)
4. Perchloric acid (30%)
5. Magnetic stirrer
6. Accurate balance weighing to ± 0.0005g
Method
1. Acidify 2mls of the urine sample to a pH of 2-3 using 30% Perchloric acid (approx 6-8 drops).
2. Dilute the sample accurately to 10 ml with the 30333 TISAB.
3. Rinse the electrodes in the 30333 TISAB buffer solution.
4. Whilst on a stirrer immerse the electrode into the sample solution and record the stable potential (E1).
5. Add a 50 microlitre aliquot of the 21333 Fluoride solution to the sample solution (continue to stir).
6. After the standard addition, record the new stable potential (E2).
Calculation
Calculate DE by E2 – E1 (mV).
The sample solution concentration is given by this equation.
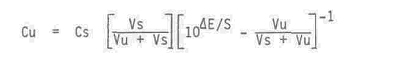
Cu = unknown solution concentration
Cs = standard concentration
Vs = volume of standard
Vu = volume of unknown
DE = change in potential (mV)
S = slope of .electrode (mV)
To attain the concentration of fluoride in the original urine sample, multiply the result calculated by 5.
